Isoquinoline
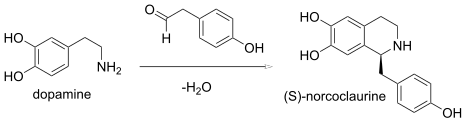
The isoquinoline ring in these natural compound derives from the aromatic amino acid tyrosine.
[3][4][5][6][7][8] Isoquinoline is a colorless hygroscopic liquid at temperatures above its melting point with a penetrating, unpleasant odor.
It crystallizes in form of platelets that have a low solubility in water but dissolve well in ethanol, acetone, diethyl ether, carbon disulfide, and other common organic solvents.
Weissgerber developed a more rapid route in 1914 by selective extraction of coal tar, exploiting the fact that isoquinoline is more basic than quinoline.
This reaction uses a benzaldehyde and aminoacetoaldehyde diethyl acetal, which in an acid medium react to form isoquinoline.
In the Bischler–Napieralski reaction an β-phenylethylamine is acylated and cyclodehydrated by a Lewis acid, such as phosphoryl chloride or phosphorus pentoxide.
In enzymology, the (S)-norcoclaurine synthase (EC 4.2.1.78) is an enzyme that catalyzes a biological Pictect-Spengler synthesis: Intramolecular aza Wittig reactions also afford isoquinolines.
Isoquinolines find many applications, including: Parkinson's disease, a slowly progressing movement disorder, is thought to be caused by certain neurotoxins.