Isotype (immunology)
Naive B cells express IgM and IgD isotypes with unmutated variable genes, which are produced from the same initial transcript following alternative splicing.
Expression of other antibody isotypes (in humans: IgG, IgA, and IgE) occurs via a process of class switching after antigen exposure.
Upon antigenic stimulation, IgM+ B cells secrete pentameric IgM antibody formed by five Ig monomers which are linked via disulfide bonds.
The pentameric structure of IgM antibodies makes them efficient at binding antigens with repetitive epitopes (e.g. bacterial capsule, viral capsid) and activation of complement cascade.
The levels of surface expression of IgD isotype has been associated with differences in B cell activation status but their role in serum is poorly understood.
[6] The IgG, IgE and IgA antibody isotypes are generated following class-switching during germinal centre reaction and provide different effector functions in response to specific antigens.
Despite the high sequence similarity (90% identical on the amino acid level), each subclass has a different half-life, a unique profile of antigen binding and distinct capacity for complement activation.
[17] sIgA has also been shown to potentiate the immune response in intestinal tissue by uptake of antigen together with the bound antibody by dendritic cells.

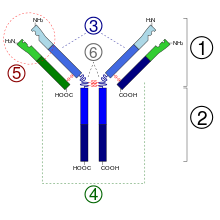
- Fab region
- Fc region
- Heavy chain (blue) with one variable (V H ) domain followed by a constant domain (C H 1), a hinge region, and two more constant (C H 2 and C H 3) domains
- Light chain (green) with one variable (V L ) and one constant (C L ) domain
- Antigen binding site (paratope)
- Hinge regions