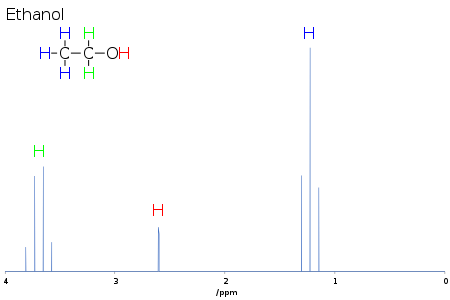
J-coupling
It is responsible for the often complex splitting of resonance lines in the NMR spectra of fairly simple molecules.
The origin of J-coupling can be visualized by a vector model for a simple molecule such as hydrogen fluoride (HF).
The selection rules of NMR spectroscopy dictate that ΔI = 1, which means that a given photon (in the radio frequency range) can affect ("flip") only one of the two nuclear spins.
One of the great conveniences of NMR spectroscopy for organic molecules is that several important lighter spin 1/2 nuclei are either monoisotopic, e.g. 31P and 19F, or have very high natural abundance, e.g. 1H.
An additional convenience is that 12C and 16O have no nuclear spin so these nuclei, which are common in organic molecules, do not cause splitting patterns in NMR.
[9] For example in the diethylthallium ion (C2H5)2Tl+, this method showed that the methyl-thallium (CH3-Tl) and methylene-thallium (CH2-Tl) coupling constants have opposite signs.
[9] The first experimental method to determine the absolute sign of a J-coupling constant was proposed in 1962 by Buckingham and Lovering, who suggested the use of a strong electric field to align the molecules of a polar liquid.
It depends on molecular orientation, but in an isotropic liquid it reduces to a number, the so-called scalar coupling.
In 1D NMR, the scalar coupling leads to oscillations in the free induction decay as well as splittings of lines in the spectrum.
[16] Independently, in October 1951, E. L. Hahn and D. E. Maxwell reported a spin echo experiment which indicates the existence of an interaction between two protons in dichloroacetaldehyde.
In the echo experiment, two short, intense pulses of radiofrequency magnetic field are applied to the spin ensemble at the nuclear resonance condition and are separated by a time interval of τ.
If the spin ensemble consists of a magnetic moment, a monotonic decay in the echo envelope is obtained.
The direct interaction between two magnetic dipoles depends on the relative position of two nuclei in such a way that when averaged over all possible orientations of the molecule it equals to zero.
In November 1951, N. F. Ramsey and E. M. Purcell proposed a mechanism that explained the observation and gave rise to an interaction of the form I1·I2.
[18] In the 1990s, direct evidence was found for the presence of J-couplings between magnetically active nuclei on both sides of the hydrogen bond.