Jelly roll fold
The name of the structure was introduced by Jane S. Richardson in 1981, reflecting its resemblance to the jelly or Swiss roll cake.
[4][8] A large number of viruses build their exterior capsids from proteins containing either a single or a double jelly roll fold.
This shared capsid architecture is thought to reflect ancient evolutionary relationships, possibly dating to before the last universal common ancestor (LUCA) of cellular life.
[12] Another group of viruses uses single jelly roll proteins in their capsids, but in the vertical rather than horizontal orientation.
These viruses are evolutionarily related to the large group of double jelly-roll viruses known as the PRD1-adenovirus viral lineage, with similar capsid architecture realized through assembly of two distinct single jelly-roll major capsid proteins expressed from distinct genes.
[12][22] Although most members of this group have icosahedral capsid geometry, a few families such as the Poxviridae and Ascoviridae have oval or brick-shaped mature virions; poxviruses such as Vaccinia undergo dramatic conformational changes mediated by highly derived double jelly roll proteins during maturation and likely derive from an icosahedral ancestor.
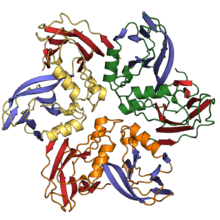
The N-terminal domain of nucleoplasmins possesses a single jelly roll fold and assembled into a pentamer.
[35][36] Cellular proteins with the double jelly roll fold include glycoside hydrolases of the DUF2961 family, peptide:N-glycosidase F (PNGases F) and peptidylglycine alpha-amidating monooxygenase.
By contrast, jelly-roll domains of DUF2961 proteins contain an insertion of short β-hairpins upstream of the G and G' strands of the double jelly roll fold.
The edges of the two sheets do not meet to form regular hydrogen bonding patterns, and so it is often not considered to be a true beta barrel,[3] though the term is in common use in describing viral capsid architecture.