Static light scattering
Static light scattering is a technique in physical chemistry that measures the intensity of the scattered light to obtain the average molecular weight Mw of a macromolecule like a polymer or a protein in solution.
By measuring the scattering intensity for many samples of various concentrations, the second virial coefficient, A2, can be calculated.
[1][2][3][4][5] Static light scattering is also commonly utilized to determine the size of particle suspensions in the sub-μm and supra-μm ranges, via the Lorenz-Mie (see Mie scattering) and Fraunhofer diffraction formalisms, respectively.
The angular dependence is required to obtain accurate measurements of both molar mass and size for all macromolecules of radius above 1–2% of the incident wavelength.
Additional details on the history and theory of MALS may be found in multi-angle light scattering.
To measure the average molecular weight directly without calibration from the light scattering intensity, the laser intensity, the quantum efficiency of the detector, and the full scattering volume and solid angle of the detector need to be known.
Since this is impractical, all commercial instruments are calibrated using a strong, known scatterer like toluene since the Rayleigh ratio of toluene and a few other solvents were measured using an absolute light scattering instrument.
Usually, detectors will have slightly different quantum efficiency, different gains, and are looking at different geometrical scattering volumes.
with n0 the refractive index of the solvent, λ the wavelength of the light source, NA the Avogadro constant, c the solution concentration, and dn/dc the change in the refractive index of the solution with change in concentration.
As described above, the radius of gyration, Rg, and the second virial coefficient, A2, are also calculated from this equation.
A simple static light scattering experiment entails the average intensity of the sample that is corrected for the scattering of the solvent will yield the Rayleigh ratio, R as a function of the angle or the wave vector q as follows:
The scattered intensity can be plotted as a function of the angle to give information on the Rg which can simply be calculated using the Guinier approximation (developed by André Guinier) as follows:
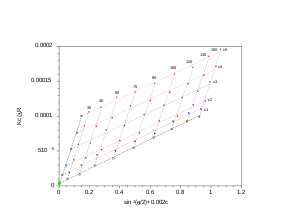
Hence a plot of the corrected Rayleigh ratio, ΔR(θ) vs sin2(θ/2) or q2 will yield a slope Rg2/3.
) as determined by static light scattering, a Zimm plot is a conventional means of deriving the parameters such as Rg, molecular mass Mw and the second virial coefficient A2.
One must note that if the material constant K is not implemented, a Zimm plot will only yield Rg.
As the angular information is available, it is also possible to obtain the radius of gyration (Rg).
The partial Zimm however, does not yield the second virial coefficient, due to the absence of the variable concentration of the sample.
The intercept of the fitted line gives the molecular mass, while the slope corresponds to the 2nd virial coefficient.
As the Debye plot is a simplification of the Zimm equation, the same limitations of the latter apply, i.e., samples should present a monodisperse nature.
For polydisperse samples, the resulting molecular mass from a static light-scattering measurement will represent an average value.
An advantage of the Debye plot is the possibility to determine the second virial coefficient.
Accurate interpretation becomes exceedingly difficult for systems with non-negligible contributions from multiple scattering.
In many commercial instruments where analysis of the scattering signal is automatically performed, the error may never be noticed by the user.
Particularly for larger particles and those with high refractive index contrast, this limits the application of standard static light scattering to very low particle concentrations.
On the other hand, for soluble macromolecules that exhibit a relatively low refractive index contrast versus the solvent, including most polymers and biomolecules in their respective solvents, multiple scattering is rarely a limiting factor even at concentrations that approach the limits of solubility.
Different implementations of cross-correlation light scattering have been developed and applied.
[12] Samples that change their properties after dilution may not be analyzed via static light scattering in terms of the simple model presented here as the Zimm equation.
A more sophisticated analysis known as 'composition-gradient static (or multi-angle) light scattering' (CG-SLS or CG-MALS) is an important class of methods to investigate protein–protein interactions, colligative properties, and other macromolecular interactions as it yields, in addition to size and molecular weight, information on the affinity and stoichiometry of molecular complexes formed by one or more associating macromolecular/biomolecular species.
In particular, static light scattering from a dilution series may be analyzed to quantify self-association, reversible oligomerization, and non-specific attraction or repulsion, while static light scattering from mixtures of species may be analyzed to quantify hetero-association.
[13] One of the main applications of static light scattering for molecular mass determination is in the field of macromolecules, such as proteins and polymers,[14][15][16] as it is possible to measure the molecular mass of proteins without any assumption about their shape.