Krische allylation
[1][2] The mechanism of the Krische allylation involves primary alcohol dehydrogenation or, when using aldehyde reactants, hydrogen transfer from 2-propanol.
[3] In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor.
In 1991, Yamamoto disclosed the first catalytic enantioselective method for carbonyl allylation, which employed a chiral boron Lewis acid-catalyst in combination with allyltrimethylsilane.
Catalytic variants of the Nozaki-Hiyama-Kishi reaction represent an alternative method for asymmetric carbonyl allylation, but stoichiometric metallic reductants are required.
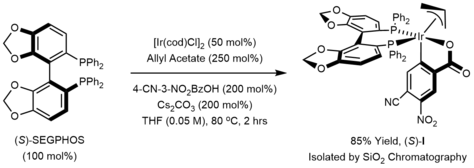
Deprotonation of the iridium hydride III provides an anionic iridium(I) species IV, which upon oxidative addition to the allyl donor forms the π-allyliridium complex V. Association of the aldehyde to the σ-allyliridium species VI triggers carbonyl addition by way of the six-centered transition structure VII to form the homoallylic alkoxide VIII.
The homoallylic alkoxide VIII is stable with respect to beta-hydride elimination due to coordination of the double bond with the metal.
Exchange with the primary alcohol reactant regenerates the iridium alkoxide I and releases the reaction product.
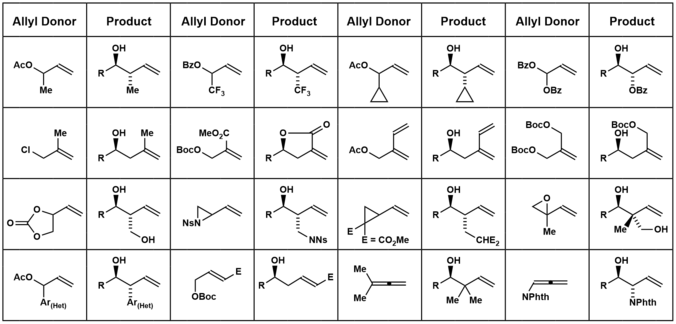
For example, total syntheses of roxaticin, bryostatin and cryptocaryol were accomplished via double Krische allylation of 1,3-propane diol.