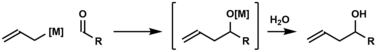
Carbonyl allylation
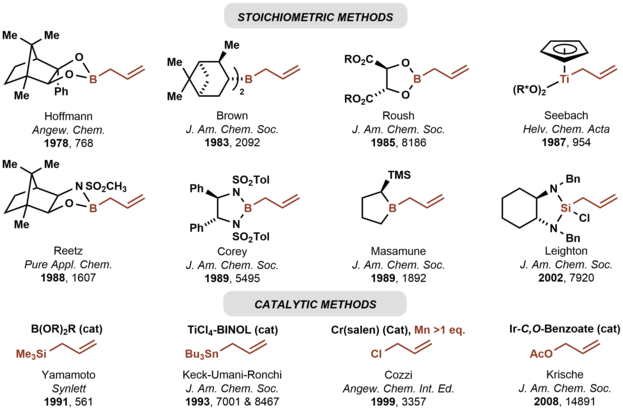
[2] In 1978, Hoffmann reported the first asymmetric carbonyl allylation using a chiral allylmetal reagent, an allylborane derived from camphor.
[9][6] Catalytic variants of the Nozaki-Hiyama-Kishi reaction represent an alternative method for asymmetric carbonyl allylation, but stoichiometric metallic reductants are required.
Carbonyl allylation has been employed in the synthesis of polyketide natural products and other oxygenated molecules with a contiguous array of stereocenters.
For example, allylstannanation of a threose-derived aldehyde affords the macrolide antascomicin B, which structurally resembles FK506 and rapamycin, and is a potent binder of FKBP12.
[12] The Krische allylation was used to prepare the polyketide (+)-SCH 351448, a macrodiolide ionophore bearing 14 stereogenic centers.