Allyl acetate
This colourless liquid is a precursor to especially allyl alcohol, which is a useful industrial intermediate.
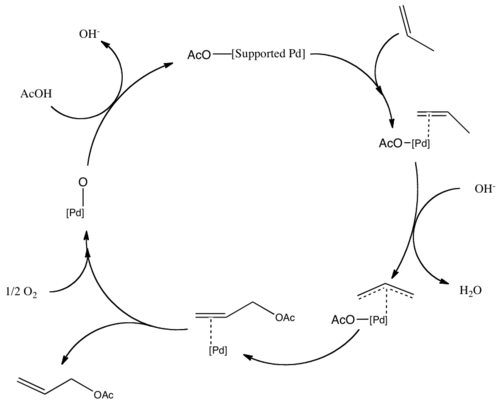
Allyl acetate is produced industrially by the gas phase reaction of propene in the presence of acetic acid using a palladium catalyst:[2][3] This method is advantageous because propene is inexpensive and a green chemical.
The mechanism for the acetoxylation follows a similar pathway, with propene forming a π-allyl bond on the palladium.
Epoxidation by hydrogen peroxide produces glycidol, which undergoes hydrolysis to glycerol.
[6] Allyl chloride is generally produced directly by the chlorination of propene.