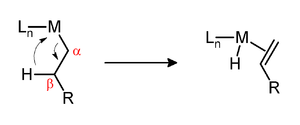
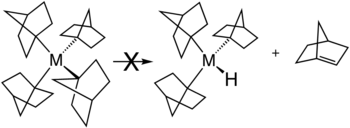β-Hydride elimination
[3] The production of branched polymers from ethylene relies on chain walking, a key step of which is β-hydride elimination.
In Hydroformylation, β-hydride elimination can act as a side reaction that influences product regioselectivity.
[4] For example, in the hydroformylation of open chain unsaturated ethers, it reverses the formation of branched metal-alkyl intermediates at high temperatures, leading to a greater yield of linear products.
In this step, a vacant site on the metal forms an agostic complex by binding a C-H bond of the alkyl (or alkoxide).
[11][12][13][14] This was suggested primarily based on the observed order of the L-type ligand in the rate law derived from kinetic studies.
The most common strategy is to employ alkyl ligands that lack hydrogen atoms at the β position.
This situation is illustrated by the stability of metal complexes containing norbornyl ligands, where the β-hydride elimination product would violate Bredt's rule.