Lanosterol synthase
[11] Due to the enzyme's role in cholesterol biosynthesis, there is interest in lanosterol synthase inhibitors as potential cholesterol-reducing drugs, to complement existing statins.
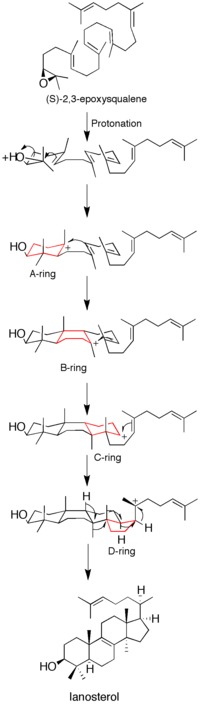
[12] Though some data on the mechanism has been obtained by the use of suicide inhibitors, mutagenesis studies, and homology modeling, it is still not fully understood how the enzyme catalyzes the formation of lanosterol.
[12] Before the acquisition of the protein's X-ray crystal structure, site-directed mutagenesis was used to determine residues key to the enzyme's catalytic activity.
[13] After acquisition of the X-ray crystal structure of the enzyme, the role of D455 as a proton donor to the substrate's epoxide was confirmed, though it was found that D455 is more likely stabilized by hydrogen bonding from two cysteine residues (C456 and C533) than from the earlier suggested histidine.
The widely popular statin drugs currently used to lower LDL (low-density lipoprotein) cholesterol function by inhibiting HMG-CoA reductase activity.
[6] Because this enzyme catalyzes the formation of precursors far upstream of (S)-2,3-epoxysqualene and cholesterol, statins may negatively influence amounts of intermediates required for other biosynthetic pathways (e.g. synthesis of isoprenoids, coenzyme Q).
Phylogenetic trees constructed from the amino acid sequences of OSCs in diverse organisms suggest a single common ancestor, and that the synthesis pathway evolved only once.
[23] The discovery of steranes including cholestane in 2.7-billion year-old shales from Pilbara Craton, Australia, suggests that eukaryotes with OSCs and complex steroid machinery were present early in earth's history.