Types of periodic tables
[2][n 1] On spiral periodic tables, "Mendeleev...steadfastly refused to depict the system as [such]...His objection was that he could not express this function mathematically.
To a lesser extent, the more involved two-dimensional arrangements do little toward solving the difficulty, and essentially the only suggestions as to modifications which are truly constructive are those centering in reflection of electronic configurations.
For convenience, periodic tables may be typified as either: 1. short; 2. triangular; 3. medium; 4. long; 5. continuous (circular, spiral, lemniscate, or helical); 6. folding; or 7. spatial.
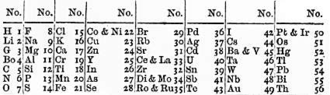
John Gladstone, a fellow chemist, objected on the basis that Newlands's table presumed no elements remained to be discovered.
Fellow English chemist Carey Foster humorously inquired of Newlands whether he had ever examined the elements according to the order of their initial letters.
Foster believed that any arrangement would present occasional coincidences, but he condemned one which placed so far apart manganese and chromium, or iron from nickel and cobalt.
[28] Advantages of this form are its aesthetic appeal, and relatively compact size; disadvantages are its width, the fact that it is harder to draw, and interpreting certain periodic trends or relationships may be more challenging compared to the traditional rectangular format.
The popularity of this form is thought to be a result of it having a good balance of features in terms of ease of construction and size, and its depiction of atomic order and periodic trends.
[43] Deming's version of a medium table, which appeared in the first edition of his 1923 textbook "General Chemistry: An Elementary Survey Emphasizing Industrial Applications of Fundamental Principles", has been credited with popularizing the 18-column form.
[44][n 6] LeRoy[45] referred to Deming's table, "this...being better known as the 'eighteen columns'-form" as representing "a very marked improvement over the original Mendeleef type as far as presentation to beginning classes is concerned."
In the first image in this section, of a so-called left step table: The elements remain positioned in order of atomic number (Z).
That being said it shows a reasonable correspondence with the Madelung energy ordering rule this being a notional sequence in which the electron shells of the neutral atoms in their ground states are filled.
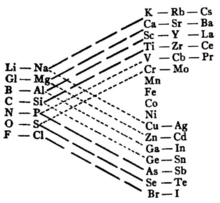
The French geologist Alexandre-Émile Béguyer de Chancourtois was the first person to make use of atomic weights to produce a classification of periodicity.
While they offer unique advantages, their complexity and customization requirements make them more suitable for specialized research, advanced education, or specific areas of study where a deeper understanding of multidimensional relationships is desired.