Limiting reagent
[1][2] The amount of product formed is limited by this reagent, since the reaction cannot continue without it.
[3] The limiting reagent must be identified in order to calculate the percentage yield of a reaction since the theoretical yield is defined as the amount of product obtained when the limiting reagent reacts completely.
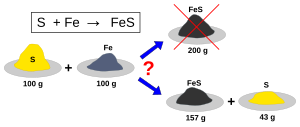
The limiting reactant is the one which can form the smallest amount of the product considered.
20.0 g of iron (III) oxide (Fe2O3) are reacted with 8.00 g aluminium (Al) in the following thermite reaction: Since the reactant amounts are given in grams, they must be first converted into moles for comparison with the chemical equation, in order to determine how many moles of Fe can be produced from either reactant.
It can be seen from the example above that the amount of product (Fe) formed from each reagent X (Fe2O3 or Al) is proportional to the quantity