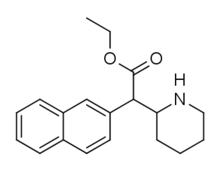
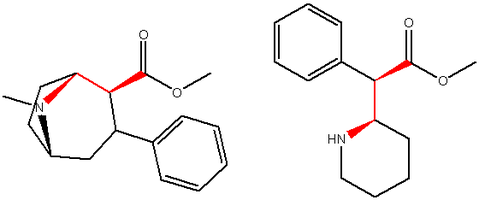
List of methylphenidate analogues
The most well known compound from this family, methylphenidate, is widely prescribed around the world for the treatment of attention deficit hyperactivity disorder (ADHD) and certain other indications.
[4][5] Dozens more phenidates and related compounds are known from the academic and patent literature, and molecular modelling and receptor binding studies have established that the aryl and acyl substituents in the phenidate series are functionally identical to the aryl and acyl groups in the phenyltropane series of drugs, suggesting that the central core of these molecules is primarily acting merely as a scaffold to correctly orientate the binding groups, and for each of the hundreds of phenyltropanes that are known, there may be a phenidate equivalent with a comparable activity profile.
Methylphenidate (and its derivatives) have two chiral centers, meaning that it, and each of its analogues, have four possible enantiomers, each with differing pharmacokinetics and receptor binding profiles.
Forms include the racemate, the enantiopure (dextro or levo) of its stereoisomers; erythro or threo (either + or -) among its diastereoisomers, the chiral isomers S,S; S,R/R,S or R,R and, lastly, the isomeric conformers (which are not absolute) of either its anti- or gauche- rotamer.
[c]) Furthermore, the energy to change between its two rotamers involves the stabilizing of the hydrogen bond between the protonated amine (of an 8.5 pKa) with the ester carbonyl resulting in reduced instances of "gauche—gauche" interactions via its favoring for activity the "anti"-conformer for putative homergic-psychostimulating pharmacokinetic properties, postulating that one inherent conformational isomer ("anti") is necessitated for the activity of the threo diastereoisomer.
[18] p-hydroxymethylphenidate displays low brain penetrability, ascribed to its phenolic hydroxyl group undergoing ionization at physiological pH.



N.B. although the cyclohexane conformation , if considering both the hydrogen on the plain bond and the implicit carbon on the dotted bond are not shown as positioned as would be for the least energy state inherent to what rules apply, internally, to the molecule in and of itself: possibility of movement between putative other ligand sites in suchwise, here regarding what circumstance allows for describing it as "flexed" thus mean it has shown tendency for change in situ depending on its environment and adjacent sites of potential interaction as against its least energy state.