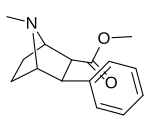
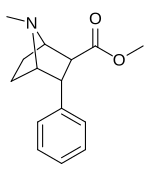
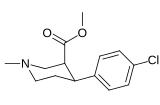
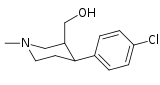
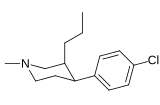
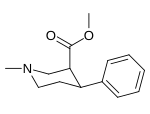
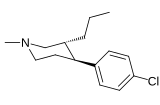
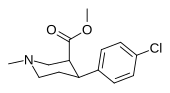
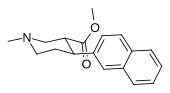
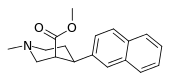
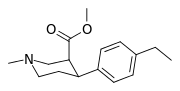
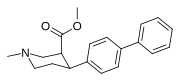
List of phenyltropanes
[c] The manner in which this sets phenyltropanes into the binding pocket at MAT is postulated as one possible explanation to account for PTs increased behavioral stimulation profile over cocaine.
Dimers of phenyltropanes, connected in their dual form using the C2 locant as altered toward a carboxamide structural configuring (in contrast and away from the usual inherent ecgonine carbmethoxy), as per Frank Ivy Carroll's patent inclusive of such chemical compounds, possibly so patented due to being actively delayed pro-drugs in vivo.
A potential disadvantage of leaving the ββ-ester unreacted is that in addition to being hydrolyzable, it can also epimerize[17] to the energetically more favorable trans configuration.
Focusing on the vicinity of the atoms @ positions A—C, the minima of electrostatic potential near atom position A (ΔVmin(A)), calculated with semi-empirical (AM1) quantum mechanics computations (superimposing the heterocyclic and phenyl rings to ascertain the least in the way of steric and conformational discrepancies) found a correlation between affinity @ DAT and ΔVmin(A): wherein the values for the latter for 32c = 0, 32g = -4, 32h = -50 & 32i = -63 kcal/mol.
In contrast to this trend, it is understood that an increasingly negative ΔVmin is correlated with an increase of strength in hydrogen bonding, which is the opposing trend for the above; this indicates that the 2β-substituents (at least for the heterocyclic class) are dominated by electrostatic factors for binding in-the-stead of the presumptive hydrogen bonding model for this substituent of the cocaine-like binding ligand.
HD-205 (Murthy et al., 2007)[25] Note the contrast to the phenylisothiocyanate covalent binding site locations as compared to the one on p-Isococ, a non-phenyltropane cocaine analogue.
WO 2004113297, Peters, Dan; Olsen, Gunnar M. & Nielsen, Elsebet Oestergaard et al., "Aza-ring derivatives and their use as monoamine neurotransmitter re-uptake inhibitors", published 2004-12-29, assigned to NeuroSearch AS U.S. patent 2,001,047,028
U.S. patent 2,001,047,028 WO 2004072075, Peters, Dan; Nielsen, Elsebet Oestergaard & Olsen, Gunnar M. et al., "Novel 8-aza-bicyclo[3.2.1]octane derivatives and their use as monoamine neurotransmitter re-uptake inhibitors", published 2004-08-26, assigned to NeuroSearch AS Unlike metal complexed PTs created with the intention of making useful radioligands, 21a & 21b were produced seeing as their η6-coordinated moiety dramatically altered the electronic character and reactivity of the benzene ring, as well as such a change adding asymmetrical molecular volume to the otherwise planar arene ring unit of the molecule.
21a was twice as potent as both cocaine and troparil in displacement of β-CFT, as well as displaying high & low affinity Ki values in the same manner as those two compounds.
21b by contrast had a one hundredfold decrease in high-affinity site binding compared to cocaine and a potency 10× less for inhibiting DA uptake.
The fact that hydrolysis of the ester leads to inactive metabolites means that this is still the main mode of deactivation for analogues that have an easily metabolised 2-ester substituent.
The attached table provides good illustration of the effect of this chemical transformation on MAT binding affinities.
[35] ɑThese values determined in Cynomolgus monkey caudate-putamen bThe radioligand used for 5-HTT was [3H]citalopram See the N-methyl paroxetine homologues cf.
The eight position nitrogen has been found to not be an exclusively necessary functional anchor for binding at the MAT for phenyltropanes and related compounds.
Sulfurs, oxygens, and even the removal of any heteroatom, leaving only the carbon skeleton of the structure at the bridged position, still show distinct affinity for the monoamine transporter cocaine-target site and continue to form an ionic bond with a measurable degree of reasonable efficacy.
Singh notes that all bridged derivatives tested displayed 2.5—104 fold higher DAT affinity than cocaine.
Note that the carbomethoxyl group is (due to constraints in synthetic processes used in the creation of this compound) alpha configured; which is not the usual, most prevalent, conformation favored for the PT cocaine-receptor binding pocket of most such sub-type of chemicals.
3-Phenyl-9-azabicyclo[3.3.1]nonane derivatives To better elucidate the binding requirements at MAT, the methylene unit on the tropane was extended by one to create the azanonane analogs.
Despite the loosened flexibility of the ring system, nitrogen constrained variants (such as were created to make the bridged class of phenyltropanes) which might better fit the rigid placement necessary to suit the spatial requirements needed in the binding pocket were not synthesized.
3-Phenyl-7-azabicyclo[2.2.1]heptane derivatives Ring-contracted analogs of phenyltropanes did not permit sufficient penetration of the phenyl into the target binding site on MAT for an affinity in the efficacious range.
Fencamfamine These compounds include transition metals in their heteroatomic conformation, unlike non-radiolabel intended chelates where their element is chosen for intrinsic affectation to binding and function, these are tagged on by a "tail" (or similar) with a sufficient spacer to remain separated from known binding properties and instead are meant to add radioactivity enough to be easily tracked via observation methods that utilize radioactivity.
As for anomalies of binding within the spectrum of the under-written kinds just mentioned: other factors not otherwise considered to account for its relatively lower potency, "compound 89c" is posited to protrude forward at the aryl place on its moiety toward the MAT ligand acceptor site in a manner detrimental to its efficacy.
However, to broach this discrepancy, decreasing of the nitrogen tether at the eight position by a single methylene unit (89d) was shown to bring the potency of the analogous compound to the expected, substantially higher, potency: The N-methyl analog of 89c having an IC50 of 1.09 ± 0.02 @ DAT & 2.47 ± 0.14 nM @ SERT; making 89c upwards of thirty-three times weaker at those MAT uptake sites.
Most any variant with a tropane locant—3-β (or α) connecting linkage differing from, e.g. longer than, a single methylene unit (i.e. "phenyl"), including alkylphenyls (see the styrene analog, first image given in example below) is more correctly a "cocaine analogue" proper, and not a phenyltropane.


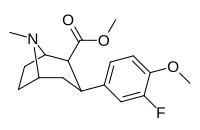

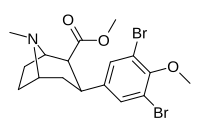



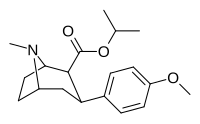
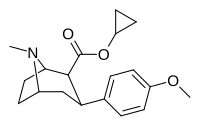

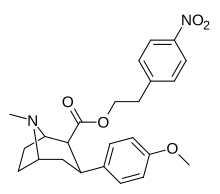
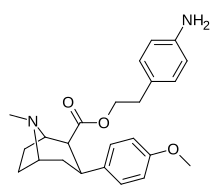



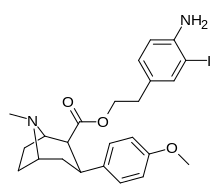
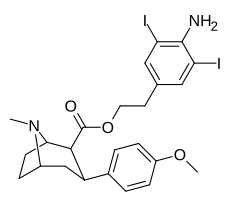

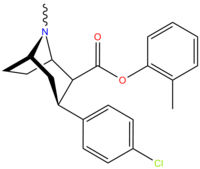



(compound model 34 )


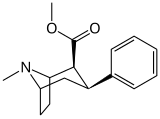
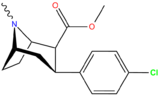




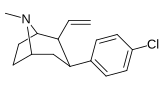

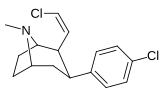


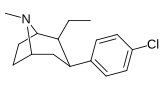
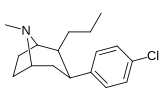



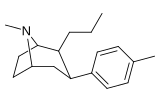





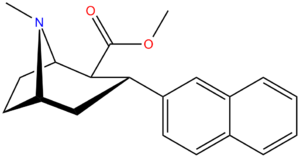


21b can be prepared from ferrocenes and perrhenate by a double ligand transfer (DLT) reaction. [ 28 ]










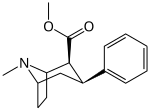




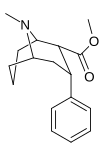

The non-carboxylic (and DAT substrate, releasing agent) variant of exo-2-phenyl-7-azabicyclo(2.2.1)heptane-1-carboxylic acid (N.B. the carboxy in the latter shares the C1 tropane position with the two carbon nitrogen containing bridge; sharing in the leftmost (R) substitution of the above depiction & unlike the placement on the tropane for either the carbmethoxy or phenyl ring of the azabornane analogues given in this section)
With the carboxy ester function removed the resultant derived compound acts as a DAT substrate drug, thus an amphetaminergic releaser of MAT & VMAT, yet similar to phenyltropanes (that usually are only re-uptake ligands) [ 41 ] cf. EXP-561 & BTQ .