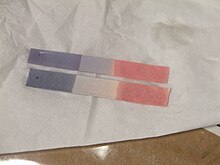
Litmus
It is often absorbed onto filter paper to produce one of the oldest forms of pH indicator, used to test materials for acidity.
[1] About 1300, the Spanish physician Arnaldus de Villa Nova began using litmus to study acids and bases.
For instance, ammonia gas, which is alkaline, turns red litmus paper blue.
[6] Some fractions of litmus were given specific names including erythrolitmin (or erythrolein), azolitmin, spaniolitmin, leucoorcein, and leucazolitmin.
[7] A recipe to make litmus out of the lichens, as outlined on a UC Santa Barbara website says:[8] Details are difficult to find because the processes were kept secret.
The lichens (preferably Lecanora tartarea and Roccella tinctoria) are ground in a solution of sodium carbonate and ammonia.
It is marketed as blue lumps, masses, or tablets, after mixing with colorless compounds such as chalk and gypsum.