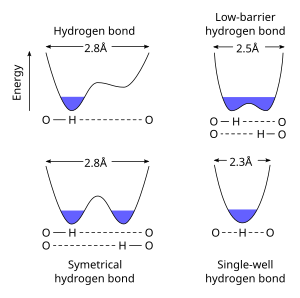
Low-barrier hydrogen bond
When pKa of the heteroatoms is closely matched, a LBHB becomes possible at a shorter distance (~2.55 Å).
LBHBs also occur on the surfaces of proteins, but are unstable due to their proximity to bulk water, and the conflicting requirements of strong salt-bridges in protein-protein interfaces.
[4] Low-barrier hydrogen bonds have been proposed to be relevant to enzyme catalysis in two types of circumstance.
[7][8] However, in 2012, a low-barrier hydrogen bond has been proposed to be involved in phosphate-arsenate discrimination for a phosphate transport protein.
[9] This finding might indicate the possibility of low-barrier hydrogen bonds playing a catalytic role in ion size selection for some very rare cases.