Lyman-alpha
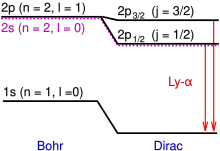
Lyman-alpha, typically denoted by Ly-α, is a spectral line of hydrogen (or, more generally, of any one-electron atom) in the Lyman series.
It is emitted when the atomic electron transitions from an n = 2 orbital to the ground state (n = 1), where n is the principal quantum number.
In hydrogen, its wavelength of 1215.67 angstroms (121.567 nm or 1.21567×10−7 m), corresponding to a frequency of about 2.47×1015 Hz, places Lyman-alpha in the ultraviolet (UV) part of the electromagnetic spectrum.
[2] Since the hydrogen Lyman-alpha radiation is strongly absorbed by the air, its observation in laboratory requires use of vacuumed spectroscopic systems.
For the same reason, Lyman-alpha astronomy is ordinarily carried out by satellite-borne instruments, except for observing extremely distant sources whose redshifts allow the line to penetrate the Earth atmosphere.