MAPK phosphatase
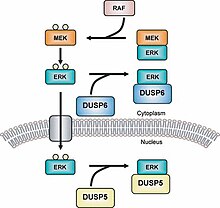
[9] There are 10[10] main MKPs that can be further broken down into three sub-classes which are representative of either their genomic structure or the type of substrate (MAPK) they bind to.
For example, MKP-1 (a type I MKP) controls gene expression by inactivating the subcellular group of MAPKs.
[17] The newest MKP, MKP-8, belongs to group I because it is located in the nuclear region of the cell[18] A recent study shows that histone deacetylase isoforms (HDAC1, -2, and -3) deacetylate MKP-1 and that this post-translational modification increases MAPK signaling and innate immune signaling.
[24] MKP-4 is another MKP that belongs to Type I and, is distinct from other MKPs in this subgroup because it is only found in placenta, kidney and embryonic liver cells.
[25] MKP-5 is a type III MKP that binds specifically to p38 and SPK/JNK and is found both in the cytoplasmic and nuclear regions of a cell.
[28] VHR is only found in lymphoid and hematopoietic cells, and it inactivates the ERK1/2 and JNKs in T-cell receptors.