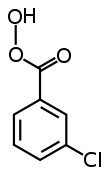
meta-Chloroperoxybenzoic acid
[1] mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material.
[2] mCPBA can be prepared by reacting m-chlorobenzoyl chloride with a basic solution of hydrogen peroxide, followed by acidification.
[3] It is sold commercially as a shelf-stable mixture that is less than 72% mCPBA, with the balance made up of m-chlorobenzoic acid (10%) and water.
[1] The peroxyacid can be purified by washing the commercial material with a sodium hydroxide and potassium phosphate solution buffered at pH = 7.5.
The purified material is reasonably stable against decomposition if stored at low temperatures in a plastic container.