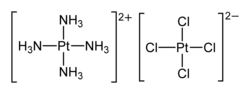
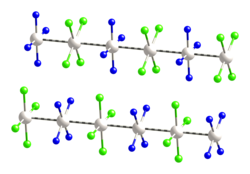
Magnus's green salt
The compound may be prepared by combining aqueous solutions of [Pt(NH3)4]2+ and [PtCl4]2−, which gives a deep green solid precipitate.
[3] Magnus's green salt is one of three compounds with the empirical formula PtCl2(NH3)2, the others being cisplatin (an important anticancer drug) and transplatin.
This difference is manifested by the solubility of the molecular complexes in water, whereas Magnus's green salt is insoluble.
Magnus's green salt occurred as an impurity in early routes to cisplatin from potassium tetrachloroplatinate.
Modern production avoids contamination by first converting the tetrachloroplatinate to potassium tetraiodoplatinate, as the iodo ligand's stronger trans effect favors the cis molecule over the polymeric salt.