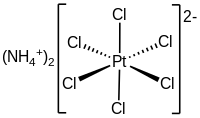
Ammonium hexachloroplatinate
The compound consists of separate tetrahedral ammonium cations and octahedral [PtCl6]2− anions.
[2] The complex is so poorly soluble that this step is employed in the isolation of platinum from ores and recycled residues.
[3] As analyzed by X-ray crystallography, the salt crystallizes in a cubic motif reminiscent of the fluorite structure.
[2] Ammonium hexachloroplatinate decomposes to yield platinum sponge when heated to high temperatures:[2][5] Dust containing ammonium hexachloroplatinate can be highly allergenic.
"Symptoms range from irritation of skin and mucous membranes to life-threatening attacks of asthma.