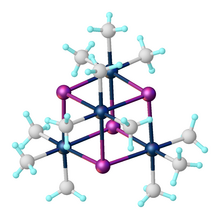
Organoplatinum chemistry
Many organoplatinum(II) complexes arise via ortho-metalation and related intramolecular C-H activation processes.
[10] The first isolated representatives were prepared from organotin halides or acids with orthometalated arylplatinum(II) compounds.
Weak acids often suffice even water and alcohol and in C-H bond activation the proton source is an alkane.
Heterogeneous catalysts based on platinum play a major role in the petrochemical industry, and it is assumed that these useful reactions proceed via surface-bound organoplatinum intermediates.
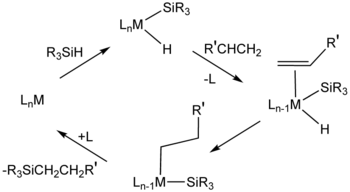
[11] Cis-dichlorobis(diethyl sulfide)platinum(II) and Karstedt's catalyst (adduct of divinyltetramethyldisiloxane and chloroplatinic acid) also catalyse hydrosilylation.
Organoplatinum compounds are implicated in the Shilov system for the conversion of methane into methyl chloride.
Strenuous efforts have been made, thus far unsuccessfully, to extend this reactivity to practical methods for functionalizing methane.
[13] For example, platinum complexes of bipyrimidine catalyze the conversion of methane, oxygen, and sulfur trioxide into methyl bisulfate.