Manganese(II) carbonate
Manganese carbonate occurs naturally as the mineral rhodochrosite but it is typically produced industrially.
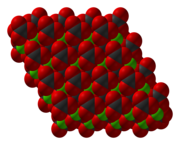
[3] MnCO3 adopts a structure like calcite, consisting of manganese(II) ions in an octahedral coordination geometry.
[4] Treatment of aqueous solutions of manganese(II) nitrate with ammonia and carbon dioxide leads to precipitation of this faintly pink solid.
Manganese carbonate decomposes with release of carbon dioxide, i.e. calcining, at 200 °C to give MnO1.88: This method is sometimes employed in the production of manganese dioxide, which is used in dry-cell batteries and for ferrites.
It is also used in multivitamins, in ceramics as a glaze colorant and flux, and in concrete stains.