Pentacarbonylhydridomanganese
[2] Of the several ways to produce this compound,[3] is the protonation of the pentacarbonyl manganate anion.
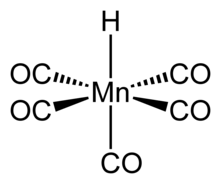
[5] HMn(CO)5 can be related to the structure of a hexacarbonyl complex such as Mn(CO)+6, and therefore has similar properties.
[6] The compound has octahedral symmetry,[7] its molecular point group is C4v and the H-Mn bond length is 1.44 ± 0.03 Å.
A common reaction involving HMn(CO)5 is substitution of the CO ligands by organophosphines, as occurs both thermally and photochemically.
HMn(CO)5 can be used to reduce olefins and other organic compounds, as well as metal halides.