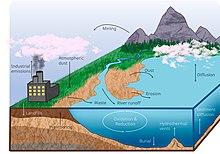
Manganese cycle
Manganese in the lithosphere enters the hydrosphere from erosion and dissolution of bedrock in rivers, in solution it then makes its way into the ocean.
The global manganese cycle is being altered by anthropogenic influences, such as mining and mineral processing for industrial use, as well as through the burning of fossil fuels.
[8] Manganese is an important soil micronutrient for plant growth, playing an essential role as a catalyst in the oxygen-evolving complex of photosystem II, a photosynthetic pathway.
[9][10] Although not all iron-reducing bacteria have the capability of reducing manganese, there is overlap in the taxa that can perform both metabolisms; these organisms are very common in a range of environmental conditions.
[11][12] Depending on the pH, organic substrate availability, and oxygen concentration, Mn can either behave as an oxidation catalyst or an electron receptor.
Estuary biogeochemistry is heavily influenced by tidal oscillations, temperature, and pH changes, and thus the manganese input into the internal cycling is variable.
[26] Overall, when looking at organism interactions with manganese, it is known that redox reactions play a key role, as well as that Mn has important biological functions, however far less is known about uptake and remineralization processes such as with iron.
Instead, soluble Mn(II) was only released into the oceans via silicate weathering on igneous rocks and supplied through hydrothermal vents.
[38] It is believed that many primary producers were anoxygenic phototrophs and took advantage of abundant hydrogen sulfide (H2S) to catalyze photosynthesis.
[40][42] The water-oxidizing complex, a key component of PSII, begins with the oxidation of Mn(II), which, along with additional evidence, strongly supports the hypothesis that manganese was a necessary step in the evolution of oxygenic photosynthesis.
Mn is utilized in many commercial products, such as fireworks, leather, paint, glass, fertilizer, animal feed, and dry cell batteries.
[46] Anthropogenic mining and mineral processing has spread Mn through three methods: wastewater discharge, industrial emissions, and releases in soils.
[48] Due to insufficient management and poor mining processes, especially in developing countries, liquid waste containing Mn can be discharged into bodies of water through anthropogenic effluents.
[53] In the Northern Hemisphere, some of the Mn pollutants released through industrial emissions are transferred to Arctic regions through atmospheric circulation, where particulates settle and accumulate in natural bodies of water.
[45] Mn is a trace element in fly ash, a residue from the use of coal for power production, which often ends up in the atmosphere, soils, and bodies of water.
[59] Due to the use of MMT as a fuel additive, motor vehicles are a significant source of Mn in the atmosphere, especially in regions of high traffic activity.