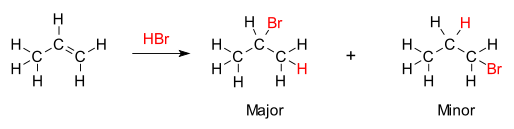
Markovnikov's rule
[1][2][3] The rule states that with the addition of a protic acid HX or other polar reagent to an asymmetric alkene, the acid hydrogen (H) or electropositive part gets attached to the carbon with more hydrogen substituents, and the halide (X) group or electronegative part gets attached to the carbon with more alkyl substituents.
[4] The same is true when an alkene reacts with water in an additional reaction to form an alcohol that involves carbocation formation.
But the other less substituted, less stable carbocation will still be formed at some concentration and will proceed to be the minor product with the opposite, conjugate attachment of X.
Early chemists discovered that the reason for the variability in the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unexpected presence of free radical ionizing substances such as peroxides.
A new method of anti-Markovnikov addition has been described by Hamilton and Nicewicz, who utilize aromatic molecules and light energy from a low-energy diode to turn the alkene into a cation radical.
In a titanium(IV) chloride-catalyzed formal nucleophilic substitution at enantiopure 1 in the scheme below, two products are formed – 2a and 2b Due to the two chiral centers in the target molecule, the carbon carrying chlorine and the carbon carrying the methyl and acetoxyethyl group, four different compounds are to be formed: 1R,2R- (drawn as 2b) 1R,2S- 1S,2R- (drawn as 2a) and 1S,2S- .