Melanopsin
9423330044ENSG00000122375ENSMUSG00000021799Q9UHM6Q9QXZ9NM_033282NM_001030015NM_001128599NM_013887NP_001025186NP_150598NP_001122071NP_038915Melanopsin is a type of photopigment belonging to a larger family of light-sensitive retinal proteins called opsins and encoded by the gene Opn4.
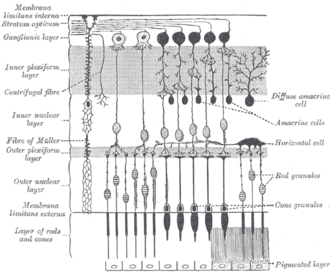
[5] In the mammalian retina, there are two additional categories of opsins, both involved in the formation of visual images: rhodopsin and photopsin (types I, II, and III) in the rod and cone photoreceptor cells, respectively.
[8] ipRGCs are photoreceptor cells which are particularly sensitive to the absorption of short-wavelength (blue) visible light and communicate information directly to the area of the brain called the suprachiasmatic nucleus (SCN), also known as the central "body clock", in mammals.
[14] A year later, researchers found that mice without any rods or cones, the cells involved in image-forming vision, still entrained to a light-dark cycle.
[15] This observation led to the conclusion that neither rods nor cones, located in the outer retina, are necessary for circadian entrainment and that a third class of photoreceptor exists in the mammalian eye.
[5] Provencio and colleagues then found in 2000 that melanopsin is also present in mouse retina, specifically in ganglion cells, and that it mediates non-visual photoreceptive tasks.
[17] They constitute a third class of photoreceptor cells in the mammalian retina, besides the already known rods and cones, and were shown to be the principal conduit for light input to circadian photoentrainment.
[19] However, non-mammalian vertebrates, including chickens and zebrafish, have another version of the melanopsin gene, Opn4x, which appears to have a distinct lineage that diverged from Opn4m about 360 million years ago.
[19] The human melanopsin gene, opn4, is expressed in ipRGCs, which comprises only 1-2% of RGCs in the inner mammalian retina, as studied by Samer Hattar and colleagues.
[16] In non-mammalian vertebrates, melanopsin is found in a wider subset of retinal cells, as well as in photosensitive structures outside the retina, such as the iris muscle of the eye, deep brain regions, the pineal gland, and the skin.
The photoresponse is selectively sensitive to short-wavelength light (peak absorption ~479 nm),[34][35] and has an intrinsic photoisomerase regeneration function that is chromatically shifted to longer wavelengths.
[7] When light with an appropriate frequency enters the eye, it activates the melanopsin contained in intrinsically photosensitive retinal ganglion cells (ipRGCs), triggering an action potential.
Consequently, stimulation of melanopsin in ipRGCs mediates behavioral and physiological responses to light, such as pupil constriction and inhibition of melatonin release from the pineal gland.
[41] Melanopsin-containing ganglion cells are thought to influence these targets by releasing the neurotransmitters glutamate and pituitary adenylate cyclase activating polypeptide (PACAP) from their axon terminals.
[18] These melanopsin-deficient mice did not completely lose their circadian rhythms, as they were still able to entrain to changing environmental stimuli, albeit more slowly than normal.
Triple-mutant mice that were rod-less, cone-less, and melanopsin-less display a complete loss in the circadian rhythms, so all three photopigments in these photoreceptors, rhodopsin, photopsin and melanopsin, are necessary for photoentrainment.
Compared to other opsins, melanopsin has an unusually long carboxy tail that contains 37 serine and threonine amino acid sites that could undergo phosphorylation.
This field has shown promise in clinical applications, including the treatment of human eye diseases such as retinitis pigmentosa and diabetes.
[52] There has been recent research on the role of melanopsin in optogenetic therapy for patients with the degenerative eye disease retinitis pigmentosa (RP).
[56] In a paper published by Ye and colleagues in 2011, melanopsin was utilized to create an optogenetic synthetic transcription device that was tested in a therapeutic setting to produce Fc-glucagon-like peptide 1 (Fc-GLP-1), a fusion protein that helps control blood glucose levels in mammals with Type II Diabetes.