Memory T cell
[3] The cross-reactivity mechanism may be important for memory T cells in the mucosal tissues since these sites have higher antigen density.
[3] For those resident in blood, bone marrow, lymphoid tissues, and spleen, homeostatic cytokines (including IL-17 and IL-15) or major histocompatibility complex II (MHCII) signaling may be more important.
This is the memory generation stage, which lasts from birth to about 20–25 years old when our immune system encounters the greatest number of new antigens.
[11] It also predicts that certain gene expression profiles would follow the on-off-on pattern during naive, effector, and memory stages.
[11] Repeated or chronic antigenic stimulation of T cells, like HIV infection, would induce elevated effector functions but reduce memory.
[14] For example, in CD4+ memory T cells, positive histone modifications mark key cytokine genes that are up-regulated during the secondary immune response, including IFNγ, IL4, and IL17A.
At early stages of infection, T cells specific for unrelated antigen are activated only by the presence of inflammation.
This happens in the inflammatory milieu resulting from microbial infection, cancer or autoimmunity in both mice and humans and occurs locally as well as systematically [25][26][27][28][29] .
[26] This phenomenon was observed predominantly in memory CD8+ T cells, which have lower sensitivity to cytokine stimulation, compared to their naive counterparts and get activated in this manner more easily.
[25] Virtual memory CD8+ T cells also display heightened sensitivity to cytokine-induced activation in mouse models, but this was not directly demonstrated in humans.
[30] In human cancerous tissues, a high number of virus-specific, not tumor-specific, CD8+ T cells was detected.
[31][32] Similarly to their CD8+ counterparts, memory and effector CD4+ T cells exhibit increased sensitivity to TCR-independent activation.
[32] TLR2 was also reported to be present on memory CD4+ T cells, which respond to their agonist by IFNγ production, even without TCR stimulation.
[32] Bystander activation plays role in the elimination of the spread of infection in its early stages and helps in tumor clearance.
[26][27][28][29] Liver injury during chronic Hepatitis B virus infection is a result of non-HBV-specific CD8+ T cell infiltration into the tissue.
[25] Furthermore, enhanced TLR2 expression was observed in joints, cartilage and bones of rheumatoid arthritis patients and the presence of its ligand, peptidoglycan, was detected in their synovial fluid.
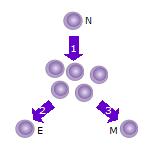
- After the naive T cell (N) encounters an antigen it becomes activated and begins to proliferate ( divide ) into many clones or daughter cells.
- Some of the T cell clones will differentiate into effector T cells (E) that will perform the function of that cell (e.g. produce cytokines in the case of helper T cells or invoke cell killing in the case of cytotoxic T cells ).
- Some of the cells will form memory T cells (M) that will survive in an inactive state in the host for a long period of time until they re-encounter the same antigen and reactivate.

In this model, memory T cells generate effector T cells, not the other way around.