Type 3 innate lymphoid cells
These cells participate in innate mechanisms on mucous membranes, contributing to tissue homeostasis, host-commensal mutualism and pathogen clearance.
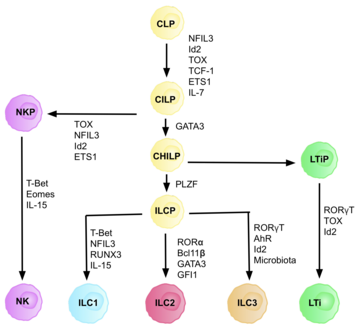
They are part of a heterogeneous group of innate lymphoid cells, which is traditionally divided into three subsets based on their expression of master transcription factors as well as secreted effector cytokines - ILC1, ILC2 and ILC3.
[2] ILC 3 family can be divided into two subgroups based on their expression of natural cytotoxicity receptors (NCRs), designated NCR+ ILC3 and NCR− ILC3.
[7] There is high heterogeneity in surface markers of ILC3 cells, with tissue-specific populations that can differ in function based on context.
In the murine model, IL-22 was found to play a role in improving the course of inflammatory bowel disease and epithelial restoration in the loss of the protective mucin barrier in the large intestine.
In the case of ILC 3, the ability to express MHC II apparently serves to maintain tolerance to commensal bacteria in the intestine.
The uptake of retinoic acid in the diet by the mother directly affects the development of LTi cells and the size of the secondary lymphoid organs of the fetus, which may have an effect at its later age.
[21] Their transcriptional and cytokine profile is very similar to Th17 cells and ILC3s can also become pathogenic in certain circumstances, contributing to disease progression and inflammation.
[23] Other studies however point to excessive ILC3 activation in both mouse models of IBD and human patients, where high levels of IL-22 were also detected.
[24] In cases of extensive invasion of pathogens to intestinal epithelium, overexpression of IL-22 and IL-17 by ILC3 might lead to excessive neutrophil influx, higher epithelial permeability and inflammation.
[27] ILC2 group has been extensively studies in relation to lung health, since dysregulation in Th2 responses is linked to asthma and other pathologies This subset is also important for tissue repairs.
ILC3 cells likely play an important role in balancing out the immune response and protecting against secondary infections in disrupted mucosal epithelia, but in cases of dysregulation their activity can lead to tissue damage.
Monocytes recruited to lungs in response to tissue damage and PAMPs have been observed to produce TNF, increasing the numbers of IL-17 secreting ILC3s, subsequently leading to neutrophil influx.
ILC3 cells have been implicated to have an important role in the pathogenesis of this disease, since they are a key population for gut homeostasis and tolerance.
In multiple sclerosis patients or EAE mouse models, levels of SCFA in fecal matter are lowered, drawing a possible link between contents of gut lumen and ILC3 mediated homeostasis.