Metallothionein
[3] MT plays a role in the protection against metal toxicity and oxidative stress, and is involved in zinc and copper regulation.
Zinc and Cadmium are tetrahedrally coordinated to cysteine residues, and each metallothionein protein molecule may bind up to 7 atoms of Zn or Cd.
[5] The biosynthesis of metallothionein appears to increase several-fold during periods of oxidative stress to shield the cells against cytotoxicity and DNA damage.
), protozoa (such as the ciliate Tetrahymena genera), plants (such as Pisum sativum, Triticum durum, Zea mays, or Quercus suber), yeast (such as Saccharomyces cerevisiae, Candida albicans, or Neurospora crassa), invertebrates (such as the nematode Caenorhabditis elegans, the insect Drosophila melanogaster, the mollusc Mytilus edulis, or the echinoderm Strongylocentrotus purpuratus) and vertebrates (such as the chicken Gallus gallus, or the mammalian Homo sapiens or Mus musculus).
The second classification was performed by Binz and Kagi in 2001, and takes into account taxonomic parameters and the patterns of distribution of Cys residues along the MT sequence.
Dimerization and oligomerization processes have been observed and attributed to several molecular mechanisms, including intermolecular disulfide formation, bridging through metals bound by either Cys or His residues on different MTs, or inorganic phosphate-mediated interactions.
Dimeric and polymeric MTs have been shown to acquire novel properties upon metal detoxification, but the physiological significance of these processes has been demonstrated only in the case of prokaryotic Synechococcus SmtA.
Strictly metal-selective MTs with metal-specific physiological functions were discovered by Dallinger et al. (1997) in pulmonate snails (Gastropoda, Mollusca).
[10] The Roman snail (Helix pomatia), for example, possesses a Cd-selective (CdMT) and a Cu-selective isoform (CuMT) involved in Cd detoxification and Cu regulation, respectively.
[12] Metallothionein has been documented to bind a wide range of metals including cadmium,[13] lead,[14] zinc, mercury, copper, arsenic, silver, etc.
[23][24] Because MTs play an important role in transcription factor regulation, defects in MT function or expression may lead to malignant transformation of cells and ultimately cancer.
[25] Studies have found increased expression of MTs in some cancers of the breast, colon, kidney, liver, skin (melanoma), lung, nasopharynx, ovary, prostate, mouth, salivary gland, testes, thyroid and urinary bladder; they have also found lower levels of MT expression in hepatocellular carcinoma and liver adenocarcinoma.
[27] Heavy metal toxicity has been proposed as a hypothetical etiology of autism, and dysfunction of MT synthesis and activity may play a role in this.
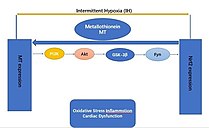
However, MT plays an important role in the anti-injury protection of the cardiovascular system, mainly in its inhibitory effect on ischemia-reperfusion injury.
[32] Transgenic mice with a deletion of any Nrf2 gene (Nrf2-KO) are highly susceptible to the cardiovascular effects of intermittent hypoxia (IH) via cardiac oxidative damage, inflammation, fibrosis, and dysfunction.
[32] Moreover, the specific overexpression in cardiomyocytes of Nrf2 (Nrf2-TG) in transgenic mice[KC1] is impervious to cardiac oxidative damage, inflammation, fibrosis, and dysfunction caused by intermittent hypoxia (IH)[KC2] .
Although not yet proven, these effects suggest that it is possible to activate PI3K/Akt/GSK3B/Fyn dependent signaling pathways through cardiac MT overexpression to prevent chronic IH-induced cardiomyopathy and downregulation of Nrf2.