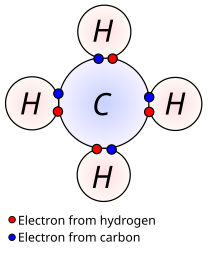
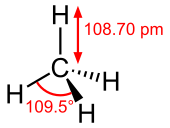Methane
The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure.
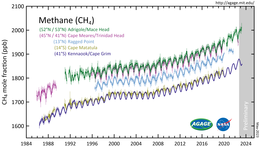
In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas.
[11] It accounted for 20% of the total radiative forcing from all of the long-lived and globally mixed greenhouse gases, according to the 2021 Intergovernmental Panel on Climate Change report.
[12] Strong, rapid and sustained reductions in methane emissions could limit near-term warming and improve air quality by reducing global surface ozone.
This is what gives Uranus and Neptune their blue or bluish-green colors, as light passes through their atmospheres containing methane and is then scattered back out.
[16] The familiar smell of natural gas as used in homes is achieved by the addition of an odorant, usually blends containing tert-butylthiol, as a safety measure.
Partial oxidation of methane to methanol (CH3OH), a more convenient, liquid fuel, is challenging because the reaction typically progresses all the way to carbon dioxide and water even with an insufficient supply of oxygen.
Liquefied natural gas (LNG) is predominantly methane (CH4) converted into liquid form for ease of storage or transport.
The fuel currently sees use in operational launch vehicles such as Zhuque-2, Vulcan and New Glenn as well as in-development launchers such as Starship, Neutron, Terran R, Nova, and Long March 9.
At high temperatures (700–1100 °C) and in the presence of a metal-based catalyst (nickel), steam reacts with methane to yield a mixture of CO and H2, known as "water gas" or "syngas": This reaction is strongly endothermic (consumes heat, ΔHr = 206 kJ/mol).
The heat needed for the reaction can also be GHG emission free, e.g. from concentrated sunlight, renewable electricity, or burning some of the produced hydrogen.
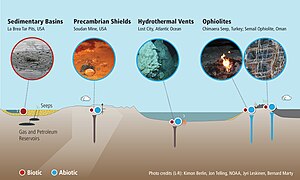
[14] Thermogenic methane occurs due to the breakup of organic matter at elevated temperatures and pressures in deep sedimentary strata.
Abiotic means that methane is created from inorganic compounds, without biological activity, either through magmatic processes[example needed] or via water-rock reactions that occur at low temperatures and pressures, like serpentinization.
[39][40] Most of Earth's methane is biogenic and is produced by methanogenesis,[41][42] a form of anaerobic respiration only known to be conducted by some members of the domain Archaea.
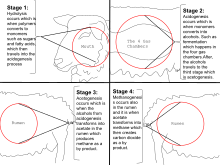
[43] Methanogens occur in landfills and soils,[44] ruminants (for example, cattle),[45] the guts of termites, and the anoxic sediments below the seafloor and the bottom of lakes.
[51] One study reported that the livestock sector in general (primarily cattle, chickens, and pigs) produces 37% of all human-induced methane.
[53] Many efforts are underway to reduce livestock methane production, such as medical treatments and dietary adjustments,[54][55] and to trap the gas to use its combustion energy.
An example of large-scale coal-to-methane gasification is the Great Plains Synfuels plant, started in 1984 in Beulah, North Dakota as a way to develop abundant local resources of low-grade lignite, a resource that is otherwise difficult to transport for its weight, ash content, low calorific value and propensity to spontaneous combustion during storage and transport.
Significant reservoirs of methane clathrates have been found in arctic permafrost and along continental margins beneath the ocean floor within the gas clathrate stability zone, located at high pressures (1 to 100 MPa; lower end requires lower temperature) and low temperatures (< 15 °C; upper end requires higher pressure).
[82] Methane "degrades air quality and adversely impacts human health, agricultural yields, and ecosystem productivity".
[84] A methane gas explosion was the cause of the Upper Big Branch coal mine disaster in West Virginia on April 5, 2010, killing 29.
[86][87] The 2015–2016 methane gas leak in Aliso Canyon, California was considered to be the worst in terms of its environmental effect in American history.
[93] Methane could be produced by a non-biological process called serpentinization[b] involving water, carbon dioxide, and the mineral olivine, which is known to be common on Mars.
[109] The discovery of methane is credited to Italian physicist Alessandro Volta, who characterized numerous properties including its flammability limit and origin from decaying organic matter.
[110] Volta was initially motivated by reports of inflammable air present in marshes by his friend Father Carlo Guiseppe Campi.
While on a fishing trip to Lake Maggiore straddling Italy and Switzerland in November 1776, he noticed the presence of bubbles in the nearby marshes and decided to investigate.
[110][111] Volta notes similar observations of inflammable air were present previously in scientific literature, including a letter written by Benjamin Franklin.
[112] Following the Felling mine disaster of 1812 in which 92 men perished, Sir Humphry Davy established that the feared firedamp was in fact largely methane.
Etymologically, the word methane is coined from the chemical suffix "-ane", which denotes substances belonging to the alkane family; and the word methyl, which is derived from the German Methyl (1840) or directly from the French méthyle, which is a back-formation from the French méthylène (corresponding to English "methylene"), the root of which was coined by Jean-Baptiste Dumas and Eugène Péligot in 1834 from the Greek μέθυ methy (wine) (related to English "mead") and ὕλη hyle (meaning "wood").
[119] Methane at scales of the atmosphere is commonly measured in teragrams (Tg CH4) or millions of metric tons (MMT CH4), which mean the same thing.