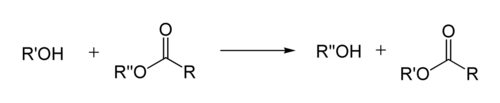
Transesterification
[1] Strong acids catalyze the reaction by donating a proton to the carbonyl group, thus making it a more potent electrophile.
If the alcohol produced by the reaction can be separated from the reactants by distillation this will drive the equilibrium toward the products.
Depending on reaction conditions ester hydrolysis and/or esterification will also occur, which results in some amount of free carboxylic acid being present.
Transesterified vegetable oil (biodiesel) was used to power heavy-duty vehicles in South Africa before World War II.
Biolipid transesterification has also been recently shown by Japanese researchers to be possible using a supercritical methanol methodology, whereby high temperature, high-pressure vessels are used to physically catalyze the biolipid/methanol reaction into fatty-acid methyl esters.