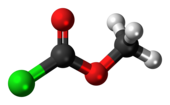
Methyl chloroformate
Methyl chloroformate can be synthesized using anhydrous methanol and phosgene.
[2] Methyl chloroformate hydrolyzes in water to form methanol, hydrochloric acid, and carbon dioxide.
The compound decomposes in heat, which can liberate hydrogen chloride, phosgene, chlorine, or other toxic gases.
[4] Methyl chloroformate is used in organic synthesis for the introduction of the methoxycarbonyl functionality to a suitable nucleophile (i.e.
[5] Methyl chloroformate forms highly flammable vapour-air mixtures.