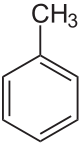
Toluene
Toluene (/ˈtɒl.juiːn/), also known as toluol (/ˈtɒl.ju.ɒl, -ɔːl, -oʊl/), is a substituted aromatic hydrocarbon[15] with the chemical formula C6H5CH3, often abbreviated as PhCH3, where Ph stands for the phenyl group.
[17][18] The compound was first isolated in 1837 through a distillation of pine oil by Pierre Joseph Pelletier and Filip Neriusz Walter, who named it rétinnaphte.
[28][29][30] Because the methyl group has greater electron-releasing properties than a hydrogen atom in the same position, toluene is more reactive than benzene toward electrophiles.
[35][36] Toluene is miscible (soluble in all proportions) with ethanol, benzene, diethyl ether, acetone, chloroform, glacial acetic acid and carbon disulfide, but immiscible with water.
[37] Toluene occurs naturally at low levels in crude oil and is a byproduct in the production of gasoline by a catalytic reformer or ethylene cracker.
Final separation and purification is done by any of the distillation or solvent extraction processes used for BTX aromatics (benzene, toluene, and xylene isomers).
Its main uses are (1) as a precursor to benzene and xylenes, (2) as a solvent for thinners, paints, lacquers, adhesives, and (3) as an additive for gasoline.
Toluene is widely used in the paint, dye, rubber, chemical, glue, printing, and pharmaceutical industries as a solvent.
Toluene is used as a cement for fine polystyrene kits (by dissolving and then fusing surfaces) as it can be applied very precisely by brush and contains none of the bulk of an adhesive.
Toluene can be used to break open red blood cells in order to extract hemoglobin in biochemistry experiments.
Toluene has also been used as a coolant for its good heat transfer capabilities in sodium cold traps used in nuclear reactor system loops.
Symptoms of toluene poisoning include central nervous system effects (headache, dizziness, drowsiness, ataxia, euphoria, tremors, hallucinations, seizures, and coma), chemical pneumonitis, respiratory depression, ventricular arrhythmias, nausea, vomiting, and electrolyte imbalances.
Inhaling high levels of toluene in a short time may cause light-headedness, nausea, or sleepiness, unconsciousness, and even death.
The US Environmental Protection Agency (EPA) states that the carcinogenic potential of toluene cannot be evaluated due to insufficient information.
People inhale toluene-containing products (e.g., paint thinner, contact cement, correction pens, model glue, etc.)
Leptodontium), Pseudeurotium zonatum, and Cladosporium sphaerospermum, and certain species of bacteria can degrade toluene using it as a source of carbon and energy.