Michael addition reaction
[1][2] It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds.
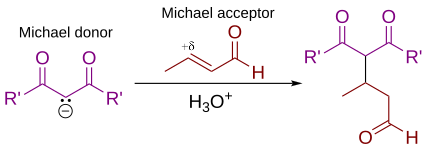
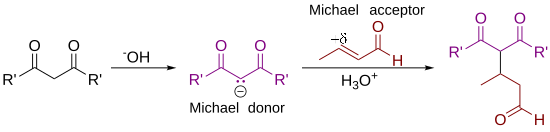
As originally defined by Arthur Michael,[7][8] the reaction is the addition of an enolate of a ketone or aldehyde to an α,β-unsaturated carbonyl compound at the β carbon.
Non-carbon nucleophiles such as water, alcohols, amines, and enamines can also react with an α,β-unsaturated carbonyl in a 1,4-addition.
[10] Some authors have broadened the definition of the Michael addition to essentially refer to any 1,4-addition reaction of α,β-unsaturated carbonyl compounds.
[11] In the reaction mechanism, there is 1 as the nucleophile:[3] Deprotonation of 1 by a base leads to carbanion 2, stabilized by its electron-withdrawing groups.
He then confirmed this assumption by reacting diethyl malonate and the ethyl ester of cinnamic acid forming the first Michael adduct:[14] In the same year Rainer Ludwig Claisen claimed priority for the invention.
[15] He and T. Komnenos had observed addition products to double bonds as side-products earlier in 1883 while investigating condensation reactions of malonic acid with aldehydes.
[14] Researchers have expanded the scope of Michael additions to include elements of chirality via asymmetric versions of the reaction.
[34][35] The 1,6-addition mechanism is similar to the 1,4-addition, with one exception being the nucleophilic attack occurring at the 𝛿 carbon of the Michael acceptor.
[35] For example, the image below shows the addition of ethylmagnesium bromide to ethyl sorbate 1 using a copper catalyst with a reversed josiphos (R,S)-(–)-3 ligand.
This particular catalyst and set of reaction conditions led to the mostly regioselective and enantioselective 1,6-Michael addition of ethyl sorbate 1 to product 3.
Cancer drugs such as ibrutinib, osimertinib, and rociletinib have an acrylamide functional group as a Michael acceptor.
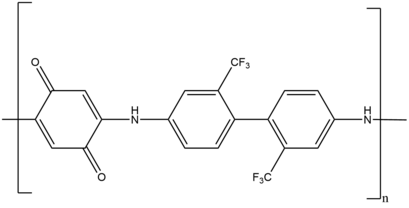
For example, linear step growth polymerization produces the redox active poly(amino quinone), which serves as an anti-corrosion coatings on various metal surfaces.