Michaelis–Arbuzov reaction
The reaction was discovered by August Michaelis in 1898,[1] and greatly explored by Aleksandr Arbuzov soon thereafter.
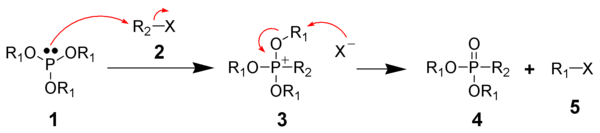
The Michaelis–Arbuzov reaction is initiated with the SN2 attack of the nucleophilic phosphorus species (1 - A phosphite) with the electrophilic alkyl halide (2) to give a phosphonium salt as an intermediate (3).
This has been supported by the observation that chiral R1 groups experience inversion of configuration at the carbon center attacked by the halide anion.
[6] Evidence also exists for a carbocation based mechanism of dealkylation similar to an SN1 reaction, where the R1 group initially dissociates from the phosphonium salt followed by attack of the anion.
[5] Phosphite esters with tertiary alkyl halide groups can undergo the reaction, which would be unexpected if only an SN2 mechanism was operating.
For example, the triaryl phosphites mentioned above generally do not react because they form stable phosphonium salts.
Since aryl groups do not undergo SN1 and SN2 type mechanisms, triaryl phosphites lack a low energy pathway for decomposition of the phosphonium salt.
Stereochemical experiments on cyclic phosphites have revealed the presence of both pentavalent phosphoranes and tetravalent phosphonium intermediates in chemical equilibrium being involved in the dealkylation step of the reaction using 31P NMR.
This is consistent with initial attack of the phosphorus reagent on the alkyl halide as the rate-determining step of the reaction.
When all of A, B and R are aryl groups, a stable phosphonium salt is formed and the reaction proceeds no further under normal conditions.
The poor availability of substituted phosphonites limits the usage of this class of reagent in the Arbuzov reaction.
They often require very little heating (45 °C) for the reaction to occur and have been known to self-isomerize without the presence of alkyl halides.
[5] The Arbuzov rearrangement generally does not admit a thiologous analogue, except when the phosphorus is substituted with strongly electron-donating groups.