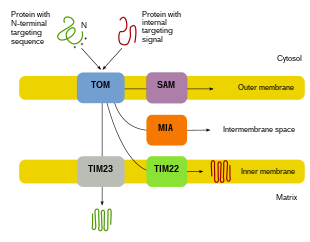
Mitochondrial biogenesis
[1][2] It was first described by John Holloszy in the 1960s, when it was discovered that physical endurance training induced higher mitochondrial content levels, leading to greater glucose uptake by muscles.
[3] Mitochondrial biogenesis is activated by numerous different signals during times of cellular stress or in response to environmental stimuli, such as aerobic exercise.
[6] The mitochondrion is a key regulator of the metabolic activity of the cell, and is also an important organelle in both production and degradation of free radicals.
[13][14] Therefore, achieving a balance between these mechanisms allows a cell to have the proper organization of its mitochondrial network during biogenesis and may have an important role in muscle adaptation to physiological stress.
[8][12][13] Multiple research studies have observed correlated increases between mitochondrial respiratory capacity with Mfn1, Mnf2, and Drp1 gene expression after endurance exercises.
[14][15] Therefore, it is supported that reorganization of the mitochondrial network in muscle cells plays an important role in response to exercise.
[4][13][15] PGC-1α, a member of the peroxisome proliferator-activated receptor gamma (PGC) family of transcriptional coactivators, is the master regulator of mitochondrial biogenesis.
[5][17][16] While there have been significant increases in mitochondria found in tissues where PGC-1α is overexpressed, as the cofactor interacts with these key transcription factors, knockout mice with disrupted PGC-1α are still viable and show normal mitochondrial abundance.
[18][5] However, a double knockout experiment of PGC-1α/β created mice that died mostly within 24 hours by defects in mitochondrial maturation of cardiac tissue.
[19][5][17] AMP-activated kinase (AMPK) also regulates mitochondrial biogenesis by phosphorylating and activating PGC-1α upon sensing an energy deficiency in muscle.
[23][24] One hypothesis for the detrimental results of aging is associated with the loss of telomeres, the end segments of chromosomes that protect genetic information from degradation.
[24][21] Deficiency of telomerase reverse transcriptase (TERT), an enzyme that plays a role in preserving telomeres, has been correlated with activated p53, a protein that suppresses PGC-1α.