Mitotic catastrophe
[1][2] Mitotic catastrophe can be induced by prolonged activation of the spindle assembly checkpoint, errors in mitosis, or DNA damage and operates to prevent genomic instability.
[4] Multiple attempts to specifically define mitotic catastrophe have been made since the term was first used to describe a temperature dependent lethality in the yeast, Schizosaccharomyces pombe, that demonstrated abnormal segregation of chromosomes.
[3] This oncosuppression is accomplished by initiating a form of cell death such as apoptosis or necrosis or by inducing cellular senescence.
[5][3] The function of this mechanism is to prevent cells from accruing genomic instability which can lead to tumorigenesis.
[3] However, the timing of cell death can vary from hours after mitosis completes to years later which has been witnessed in human tissues treated with radiotherapy.
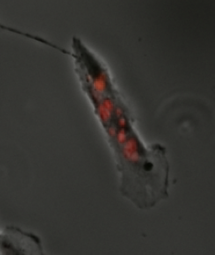
[3] Another usage of the term mitotic catastrophe is to describe a mode of cell death that occurs during mitosis.
[3] When the spindle assembly checkpoint is activated, it arrests the cell in mitosis until all chromosomes are properly attached and aligned.
[8] Normally, activation of the anaphase promoting complex leads to the separation of sister chromatids and the cell exiting mitosis.
[9] Unattached kinetochores promote the formation of the mitotic checkpoint complex which is composed of four different proteins known as Mad2, Cdc20, BubR1, and Bub3 in humans.
[11] Cyclin B1 works with its binding partner CDK1 to control this progression and the complex is known as the mitotic promoting factor.
[11] Normally, cyclin B1 degradation is initiated by the anaphase promoting complex after all of the kinetochores have been properly attached by mitotic spindle fibers.
[16] However, when there are more than two centrosomes present in mitosis they can pull chromosomes in incorrect directions resulting in daughter cells that are inviable.
[3] High levels of DNA damage that are not repaired before the cell enters mitosis can result in a mitotic catastrophe.
[3] If mitotic catastrophe fails for cells whose genome has become unstable they can propagate uncontrollably and potentially become tumorigenic.
[4] Furthermore, doses of DNA damaging drugs lower than lethal levels have been shown to induce mitotic catastrophe.
[3] Cancer therapies can induce mitotic catastrophe by either damaging the cells DNA or inhibiting spindle assembly.