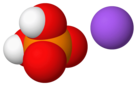
Monosodium phosphate
It consists of sodium cations (Na+) and dihydrogen phosphate anions (H2PO−4).
The pH of such formulations is generally adjusted by mixtures of various sodium phosphates, such as this salt.
[clarification needed] It is added in animal feed, toothpaste, and evaporated milk.
Monosodium phosphate is used to detect the presence of magnesium ions in salts.
Formation of a white precipitate on the addition of ammonium chloride, ammonium hydroxide and monosodium phosphate to an aqueous or dilute HCl solution of the salt indicates presence of magnesium ions.