Vanadyl sulfate
This hygroscopic blue solid is one of the most common sources of vanadium in the laboratory, reflecting its high stability.
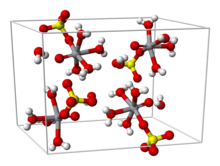
[2] Vanadyl sulfate is most commonly obtained by reduction of vanadium pentoxide with sulfur dioxide: From aqueous solution, the salt crystallizes as the pentahydrate, the fifth water is not bound to the metal in the solid.
Viewed as a coordination complex, the ion is octahedral, with oxo, four equatorial water ligands, and a monodentate sulfate.
Hydrated forms, also rare, include hexahydrate (stanleyite), pentahydrates (minasragrite, orthominasragrite,[9] and anorthominasragrite) and trihydrate - bobjonesite.
[11] Vanadyl sulfate has been extensively studied in the field of diabetes research as a potential means of increasing insulin sensitivity.