NF-κB
NF-κB is found in almost all animal cell types and is involved in cellular responses to stimuli such as stress, cytokines, free radicals, heavy metals, ultraviolet irradiation, oxidized LDL, and bacterial or viral antigens.
Incorrect regulation of NF-κB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development.
[8][9][10][11][12][13] NF-κB was discovered by Ranjan Sen in the lab of Nobel laureate David Baltimore via its interaction with an 11-base pair sequence in the immunoglobulin light-chain enhancer in B cells.
[14] Later work by Alexander Poltorak and Bruno Lemaitre in mice and Drosophila fruit flies established Toll-like receptors as universally conserved activators of NF-κB signalling.
These works ultimately contributed to awarding of the Nobel Prize to Bruce Beutler and Jules A. Hoffmann, who were the principal investigators of those studies.
[20][21] Indeed, this confounds the interpretation of p105-knockout studies, where the genetic manipulation is removing an IκB (full-length p105) and a likely repressor (p50 homodimers) in addition to a transcriptional activator (the RelA-p50 heterodimer).
The sequencing of the genomes of the mosquitoes A. aegypti and A. gambiae, and the fruitfly D. melanogaster has allowed comparative genetic and evolutionary studies on NF-κB.
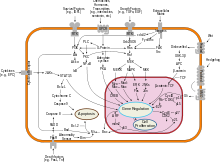
Known inducers of NF-κB activity are highly variable and include reactive oxygen species (ROS), tumor necrosis factor alpha (TNFα), interleukin 1-beta (IL-1β), bacterial lipopolysaccharides (LPS), isoproterenol, cocaine, endothelin-1 and ionizing radiation.
[28] Many bacterial products and stimulation of a wide variety of cell-surface receptors lead to NF-κB activation and fairly rapid changes in gene expression.
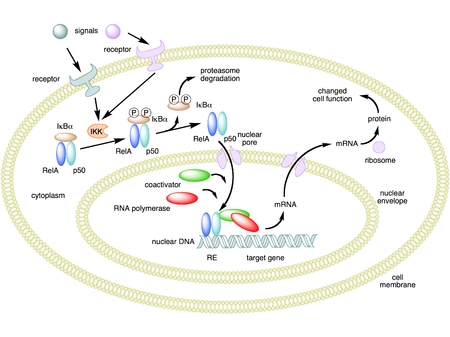
Although homodimers of p50 and p52 are, in general, repressors of κB site transcription, both p50 and p52 participate in target gene transactivation by forming heterodimers with RelA, RelB, or c-Rel.
[36][37] IκBδ degradation in response to developmental stimuli, such as those transduced through LTβR, potentiate NF-κB dimer activation in a NIK dependent non-canonical pathway.
IKK is composed of a heterodimer of the catalytic IKKα and IKKβ subunits and a "master" regulatory protein termed NEMO (NF-κB essential modulator) or IKKγ.
[45] A select set of cell-differentiating or developmental stimuli, such as lymphotoxin β-receptor (LTβR), BAFF or RANKL, activate the non-canonical NF-κB pathway to induce NF-κB/RelB:p52 dimer in the nucleus.
In this pathway, activation of the NF-κB inducing kinase (NIK) upon receptor ligation led to the phosphorylation and subsequent proteasomal processing of the NF-κB2 precursor protein p100 into mature p52 subunit in an IKK1/IKKa dependent manner.
Most intriguingly, a recent study identified that TNF-induced canonical signalling subverts non-canonical RelB:p52 activity in the inflamed lymphoid tissues limiting lymphocyte ingress.
[48] Mechanistically, TNF inactivated NIK in LTβR‐stimulated cells and induced the synthesis of Nfkb2 mRNA encoding p100; these together potently accumulated unprocessed p100, which attenuated the RelB activity.
Through a cascade of phosphorylation events, the kinase complex is activated and NF-κB is able to enter the nucleus to upregulate genes involved in T-cell development, maturation, and proliferation.
Current studies suggest that NF-κB is important for learning and memory in multiple organisms including crabs,[11][12] fruit flies,[55] and mice.
Many NF-κB target genes that may be important for plasticity and learning include growth factors (BDNF, NGF)[60] cytokines (TNF-alpha, TNFR)[61] and kinases (PKAc).
[66] Some of the DNA-binding activity noted under certain conditions (particularly that reported as constitutive) appears to result from Sp3 and Sp4 binding to a subset of κB enhancer sequences in neurons.
In the final analysis, the role of NF-κB in neurons remains opaque due to the difficulty of measuring transcription in cells that are simultaneously identified for type.
In cancer, proteins that control NF-κB signaling are mutated or aberrantly expressed, leading to defective coordination between the malignant cell and the rest of the organism.
[90] NF-κB is increasingly expressed with obesity and aging,[91] resulting in reduced levels of the anti-inflammatory, pro-autophagy, anti-insulin resistance protein sirtuin 1.
NF-κB and interleukin 1 alpha mutually induce each other in senescent cells in a positive feedback loop causing the production of senescence-associated secretory phenotype (SASP) factors.
[95] This effect may be explained, in part, by the finding that reduction of NF-κB reduces the production of mitochondria-derived reactive oxygen species that can damage DNA.
The proteosome inhibitor Bortezomib broadly blocks this activity and is approved for treatment of NF-κB driven Mantle Cell Lymphoma and Multiple Myeloma.
The drug denosumab acts to raise bone mineral density and reduce fracture rates in many patient sub-groups by inhibiting RANKL.
[120] However the studies purporting its benefit use abnormally high doses in the millimolar range (similar to the extracellular potassium concentration), which are unlikely to be achieved in humans.
[121] It has been shown that administration of BAY 11-7082 rescued renal functionality in diabetic-induced Sprague-Dawley rats by suppressing NF-κB regulated oxidative stress.
[123] The biological target of iguratimod, a drug marketed to treat rheumatoid arthritis in Japan and China, was unknown as of 2015, but the primary mechanism of action appeared to be preventing NF-κB activation.