NacNac
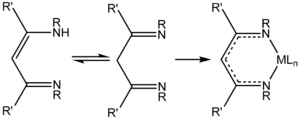
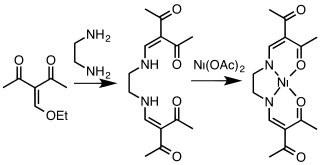
[1] Acetylacetone and related 1,3-diketones condense with primary alkyl- or arylamines resulting in replacement of the carbonyl oxygen atoms with NR groups, where R = aryl, alkyl.
To prepare 1,3-diketimines from bulky amines, e.g. 2,4,6-trimethylanilines, prolonged reaction times are required.
Deprotonation of HNacNac compounds affords anionic bidentate ligands that form a variety of coordination complexes.
[3] Unlike the situation for the acetylacetonates, the steric properties of the coordinating atoms in NacNac− ligands is adjustable by changes in the R substituent.
Attachment to a metal center is usually carried out by initial deprotonation of HNacNac with n-butyllithium; the lithium derivative is then treated with a metal chloride to eliminate lithium chloride.