Diimine
[1] Diimines are prepared by condensation reactions where a dialdehyde or diketone is treated with amine and water is eliminated.
An example is glyoxal-bis(mesitylimine), a yellow solid that is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal.
[5] For example, acetylacetone (2,4-pentanedione) and a primary alkyl- or arylamine will react, typically in acidified ethanol, to form a diketimine.
1,3-Diketimines are often referred to as HNacNac, a modification of the abbreviation Hacac for the conjugate acid of acetylacetone.
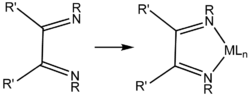
Substituted α-diimine ligands are useful in the preparation of post-metallocene catalysts, which are used for the polymerization of alkenes.