Nandrolone
[2][12][8][13] Nandrolone esters are used in the treatment of anemias, cachexia (muscle wasting syndrome), osteoporosis, breast cancer, and for other indications.
[8][13][14] Side effects of nandrolone esters include symptoms of masculinization like acne, increased hair growth, and voice changes.
[8] In addition to their medical use, nandrolone esters are used to improve physique and performance, and are said to be the most widely used anabolic steroid for such purposes.
[8] Nandrolone esters are used clinically, although increasingly rarely, for people in catabolic states with major burns, cancer, and AIDS, and an ophthalmological formulation was available to support cornea healing.
[18]: 134 The positive effects of nandrolone esters include muscle growth, appetite stimulation and increased red blood cell production,[medical citation needed] and bone density.
[20] Nandrolone theoretically may produce erectile dysfunction as a side effect, although there is no clinical evidence to support this notion at present.
[21][failed verification] Side effects of high doses of nandrolone may include cardiovascular toxicity as well as hypogonadism and infertility.
[citation needed] In addition to its AR agonistic activity, unlike many other anabolic steroids, nandrolone is also a potent progestogen.
[25] This is attributed to the fact that whereas testosterone is potentiated via conversion into dihydrotestosterone (DHT) in androgenic tissues, the opposite is true with nandrolone and similar anabolic steroids (i.e., other 19-nortestosterone derivatives).
[28][33][20] Nandrolone has very low affinity for human serum sex hormone-binding globulin (SHBG), about 5% of that of testosterone and 1% of that of DHT.
[2][12] Nandrolone is an endogenous intermediate in the production of estradiol from testosterone via aromatase in mammals including humans and is present in the body naturally in trace amounts.
Notable examples include the non-17α-alkylated trenbolone and the 17α-alkylated ethylestrenol (ethylnandrol) and metribolone (R-1881), as well as the 17α-alkylated designer steroids norboletone and tetrahydrogestrinone (THG).
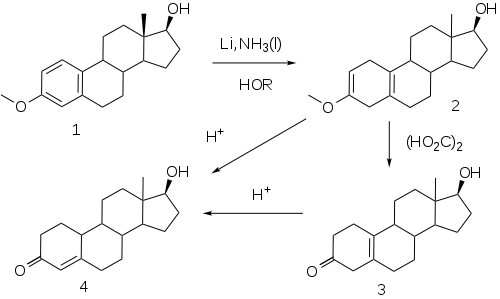
[46] The elaboration of a method for the reduction of aromatic rings to the corresponding dihydrobenzenes under controlled conditions by A. J. Birch opened a convenient route to compounds related to the putative 19-norprogesterone.
This reaction, now known as the Birch reduction,[51] is typified by the treatment of the monomethyl ether of estradiol (1) with a solution of lithium metal in liquid ammonia in the presence of alcohol as a proton source.
Rxn of the intermediate with the proton source leads to a dihydrobenzene; a special virtue of this sequence in steroids is the fact that the double bind at 2 is in effect becomes an enol ether moiety.
[60] Heavy consumption of the essential amino acid lysine (as indicated in the treatment of cold sores) has allegedly shown false positives in some and was cited by American shotputter C. J.
In October 2007, three-time Olympic gold medalist for track and field Marion Jones admitted to use of the drug, and was sentenced to six months in jail for lying to a federal grand jury in 2000.
They were intensively studied for osteoporosis, and increased calcium uptake and decreased bone loss, but caused virilization in about half of the women who took them and were mostly abandoned for this use when better drugs like the bisphosphonates became available.