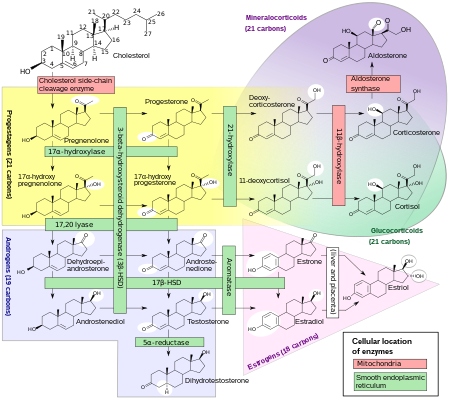
Pregnenolone
Pregnenolone and its 3β-sulfate, pregnenolone sulfate, like dehydroepiandrosterone (DHEA), DHEA sulfate, and progesterone, belong to the group of neurosteroids that are found in high concentrations in certain areas of the brain, and are synthesized there.
[4][5] Pregnenolone is involved in a natural negative feedback loop against CB1 receptor activation in animals.
Pregnenolone has been found to bind with high, nanomolar affinity to microtubule-associated protein 2 (MAP2) in the brain.
[7][8] Although pregnenolone itself does not possess these activities, its metabolite pregnenolone sulfate is a negative allosteric modulator of the GABAA receptor[9] as well as a positive allosteric modulator of the NMDA receptor.
[10][11] In addition, pregnenolone sulfate has been shown to activate the transient receptor potential M3 (TRPM3) ion channel in hepatocytes and pancreatic islets causing calcium entry and subsequent insulin release.
[23][24][25] Conversely, medical castration has been found to partially suppress pregnenolone levels in premenopausal women.
[26][27] Similarly, an adrenalectomized premenopausal woman showed incompletely diminished circulating pregnenolone levels.
[29][30] The compound contains ketone and hydroxyl functional groups, two methyl branches, and a double bond at C5, in the B cyclic hydrocarbon ring.