Nebulizer
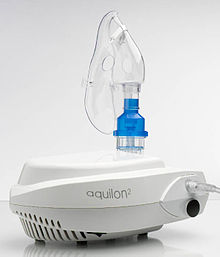
They use oxygen, compressed air or ultrasonic power to break up solutions and suspensions into small aerosol droplets that are inhaled from the mouthpiece of the device.
In addition, another trial found that a MDI (with spacer) had a lower required dose for clinical result compared to a nebulizer.
By using a nebulizer, calcium gluconate is delivered to the lungs as an aerosol to counteract the toxicity of inhaled HF vapors.
Currently there seems to be a tendency among physicians to prefer prescription of a pressurized Metered Dose Inhaler (pMDI) for their patients, instead of a jet nebulizer that generates a lot more noise (often 60 dB during use) and is less portable due to a greater weight.
However, jet nebulizers are commonly used in hospitals for patients who have difficulty using inhalers, such as in serious cases of respiratory disease, or severe asthma attacks.
[13] The medical company Boehringer Ingelheim also invented a device named Respimat Soft Mist Inhaler in 1997.
The average droplet size in the mist was measured to 5.8 micrometers, which could indicate some potential efficiency problems for the inhaled medicine to reach the lungs.
This technology is more efficient than having a vibrating piezoelectric element at the bottom of the liquid reservoir, and thereby shorter treatment times are also achieved.
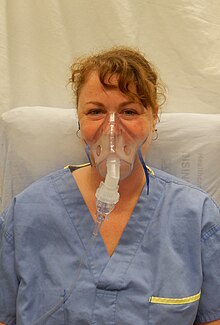
The mouthpiece, however, is sometimes replaced with a face mask, similar to that used for inhaled anesthesia, for ease of use with young children or the elderly.
Pediatric masks are often shaped like animals such as fish, dogs or dragons to make children less resistant to nebulizer treatments.
But mouthpieces are preferable if patients are able to use them since face-masks result in reduced lung delivery because of aerosol losses in the nose.
[11] After use with corticosteroid, it is theoretically possible for patients to develop a yeast infection in the mouth (thrush) or hoarseness of voice (dysphonia), although these conditions are clinically very rare.
[29] In 1956, a technology competing against the nebulizer was launched by Riker Laboratories (3M), in the form of pressurized metered-dose inhalers, with Medihaler-iso (isoprenaline) and Medihaler-epi (epinephrine) as the two first products.
[30] In these devices, the drug is cold-fill and delivered in exact doses through some special metering valves, driven by a gas propellant technology (i.e. Freon or a less environmentally damaging HFA).
[17] Some of the first models of electronic cigarettes featured an ultrasonic wave nebulizer (having a piezoelectric element vibrating and creating high-frequency ultrasound waves, to cause vibration and atomization of liquid nicotine) in combination with a vapouriser (built as a spray nozzle with an electric heating element).
[32] The most common type of electronic cigarettes currently sold, however, omit the ultrasonic wave nebulizer, as it was not found to be efficient enough for this kind of device.
Instead, the electronic cigarettes now use an electric vaporizer, either in direct contact with the absorbent material in the "impregnated atomizer," or in combination with the nebulization technology related to a "spraying jet atomizer" (in the form of liquid droplets being out-sprayed by a high-speed air stream, that passes through some small venturi injection channels, drilled in a material absorbed with nicotine liquid).