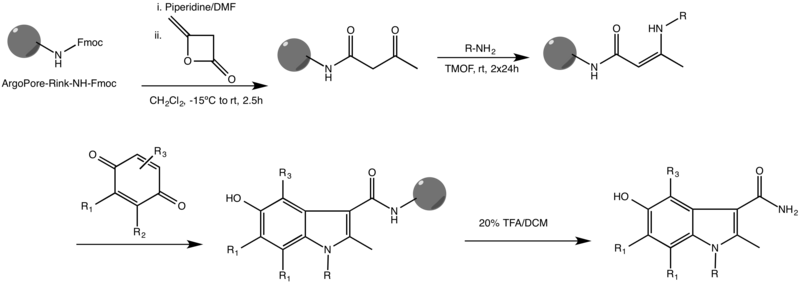
Nenitzescu indole synthesis
The Nenitzescu indole synthesis is a chemical reaction that forms 5-hydroxyindole derivatives from benzoquinone and β-aminocrotonic esters.
[3] The synthesis is particularly interesting because indoles are the foundation for a number of biochemically important molecules, including neurotransmitters and a new class of antitumor compounds.
[4] The mechanism of a Nenitzescu reaction consists of a Michael addition, followed by a nucleophilic attack by the enamine pi bond, and then an elimination.
Other solid-phase indole syntheses were also reported, some of which use different scaffolds and metal catalysts to drive the reaction to completion.
In a review article, Taber et al. categorize these reactions into nine basic types of indole syntheses: Fischer, Mori, Hemetsberger, Buchwald, Sundberg, Madelung, Nenitzescu, van Leusen and Kanematsu.