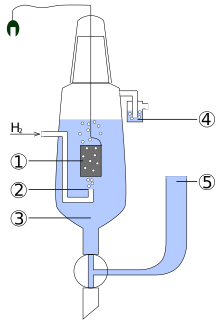
Standard hydrogen electrode
General equation for a reduction reaction: The reaction quotient (Qr) of the half-reaction is the ratio between the chemical activities (a) of the reduced form (the reductant, ared) and the oxidized form (the oxidant, aox).
Considering the 2 H+ / H2 redox couple: at chemical equilibrium, the ratio Qr of the reaction products by the reagents is equal to the equilibrium constant K of the half-reaction: where More details on managing gas fugacity to get rid of the pressure unit in thermodynamic calculations can be found at thermodynamic activity#Gases.
Meanwhile the general SHE equation can also be applied to other thermodynamic systems with different mole fraction or total pressure of hydrogen.
The electrode is immersed in the acidic solution and pure hydrogen gas is bubbled over its surface.
The concentration of both the reduced and oxidised forms of hydrogen are maintained at unity.
by definition in the case of the SHE, The Nernst equation for the SHE becomes: Simply neglecting the pressure unit present in
the equation simplifies to: This last equation describes the straight line with a negative slope of -0.0591 volt/ pH unit delimiting the lower stability region of water in a Pourbaix diagram where gaseous hydrogen is evolving because of water decomposition.
where: Note: as the system is at chemical equilibrium, hydrogen gas, H2(g), is also in equilibrium with dissolved hydrogen, H2(aq), and the Nernst equation implicitly takes into account the Henry's law for gas dissolution.
Therefore, there is no need to independently consider the gas dissolution process in the system, as it is already de facto included.
During the early development of electrochemistry, researchers used the normal hydrogen electrode as their standard for zero potential.
Inorganic ions that can be reduced to a lower valency state at the electrode also have to be avoided (e.g., Fe3+, CrO2−4).
A number of organic substances are also reduced by hydrogen on a platinum surface, and these also have to be avoided.
Cations that can be reduced and deposited on the platinum can be source of interference: silver, mercury, copper, lead, cadmium and thallium.
Substances that can inactivate ("poison") the catalytic sites include arsenic, sulfides and other sulfur compounds, colloidal substances, alkaloids, and material found in biological systems.