Nickel(II) carbonate
[3] The hexahydrate NiCO3•6H2O is claimed to form upon electrolysis of nickel metal under an atmosphere of carbon dioxide.
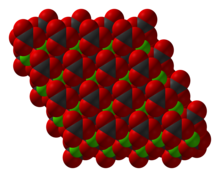
[4] NiCO3 adopts a structure like calcite, consisting of nickel in an octahedral coordination geometry.
Also known as the mineral hellyerite, the solid consists of [Ni2(CO3)2(H2O)8] subunits with an extra water of hydration.
[6] Nickel carbonates are hydrolyzed upon contact with aqueous acids to give solutions containing the ion [Ni(H2O)6]2+, liberating water and carbon dioxide in the process.
Calcining (heating to drive off CO2 and water) of these carbonates gives nickel(II) oxide: The nature of the resulting oxide depends on the nature of the precursor.