Niobium(V) ethoxide
[4] Metal alkoxides rarely adopt monomeric structures, and niobium(V) ethoxide is no exception.
[5] Subsequent crystallographic analysis established that the methoxide and isopropoxides of niobium adopt bioctahedral structures.
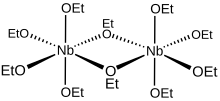
[6] From a geometric perspective, the ten ethoxide ligand oxygen atoms of the Nb2(OEt)10 molecule in solution define a pair of octahedra sharing a common edge with the two niobium atoms located at their centres.
From a bonding perspective, each niobium centre is surrounded octahedrally by four monodentate and two bridging ethoxide ligands.
The oxygen atoms of the bridging ethoxides are each bonded to both niobium centres, and these two ligands are cis to one another within the coordination sphere.